What Quality Standards Should a Supplier Meet?
Good Manufacturing Practices (GMP) Compliance
When selecting an astragalus extract powder supplier, adherence to Good Manufacturing Practices (GMP) is essential. GMP compliance confirms that the facility follows rigorous quality control protocols at every stage of production. This encompasses detailed documentation, strict hygiene standards, regular equipment calibration, and comprehensive staff training. A GMP-certified supplier not only meets regulatory requirements but also demonstrates a commitment to safety, consistency, and product integrity. Such practices minimize contamination risks and ensure that the astragalus root powder is produced under controlled and reproducible conditions. Furthermore, GMP adherence involves systematic auditing and continuous improvement processes, which help maintain high operational standards and facilitate compliance with international regulations. By choosing a GMP-compliant supplier, companies mitigate risks related to product recalls, regulatory non-conformance, and inconsistent quality, thereby safeguarding their brand reputation and consumer trust.
Standardization and Potency Assurance
Reputable suppliers of astragalus root extract powder and astragalus root powder should offer standardized products with guaranteed potency. This involves systematic testing to verify that each batch contains a consistent and specified concentration of active compounds, particularly astragalosides. Standardization supports manufacturers in creating reliable end-products with uniform therapeutic effects. Suppliers should provide comprehensive Certificates of Analysis (COA) for every batch, detailing the precise levels of key constituents. This transparency allows buyers to validate quality and incorporate trusted ingredients into their formulations. Additionally, advanced analytical methods such as High-Performance Liquid Chromatography (HPLC) are often employed to ensure accuracy in potency measurement. Consistency in active ingredient levels is critical for efficacy, especially in nutraceutical and pharmaceutical applications where dosage precision directly impacts health outcomes.
Traceability and Sustainability Practices
A dependable organic astragalus root extract supplier must implement end-to-end traceability systems. These systems track raw materials from cultivation through extraction, ensuring full transparency and quality control at each step. Furthermore, sustainable and ethical sourcing practices are increasingly critical. Suppliers who emphasize environmental responsibility, soil health, and ethical harvesting not only contribute to ecological preservation but also align with modern consumer expectations. Such commitments reflect a long-term, holistic approach to quality and corporate responsibility. Traceability also helps quickly address any quality deviations and reinforces accountability across the supply chain. Certifications such as USDA Organic, FairWild, or other third-party validations further demonstrate a supplier’s dedication to sustainable and socially responsible practices, enhancing credibility and market competitiveness.

Third-Party Testing and Certification for Astragalus Extract Powder
Independent Laboratory Analysis
Reputable suppliers of astragalus extract powder should prioritize independent laboratory testing to validate product quality. These third-party analyses are critical for verifying purity, potency, and overall safety, including screenings for heavy metals, pesticides, and microbial contaminants. Trusted suppliers conduct these tests routinely and make the results easily accessible to customers. This transparency not only ensures compliance with stringent international standards but also reinforces consumer confidence in the product’s reliability and efficacy.
Organic Certification
For manufacturers requiring organic astragalus root extract powder, certification from authoritative bodies such as USDA Organic or EU Organic is indispensable. These certifications confirm that the astragalus has been cultivated and processed without synthetic pesticides, fertilizers, or GMOs, adhering to strict organic farming protocols. Buyers should always verify that the supplier’s certification is up-to-date and specifically applies to the products they are purchasing, ensuring authenticity and compliance with organic regulations.
Quality Management System Certifications
Suppliers holding ISO certifications, especially ISO 9001, reflect a structured approach to quality management and continuous improvement. This certification underscores a supplier's dedication to maintaining high standards in production, documentation, and customer satisfaction. For astragalus extract used in food or supplements, ISO 22000 certification is equally important, as it focuses on food safety management systems, ensuring the product meets safety and quality expectations throughout the supply chain.
Global Market Trends in Astragalus Extract Powder Supply
Increasing Demand in Nutraceutical and Pharmaceutical Industries
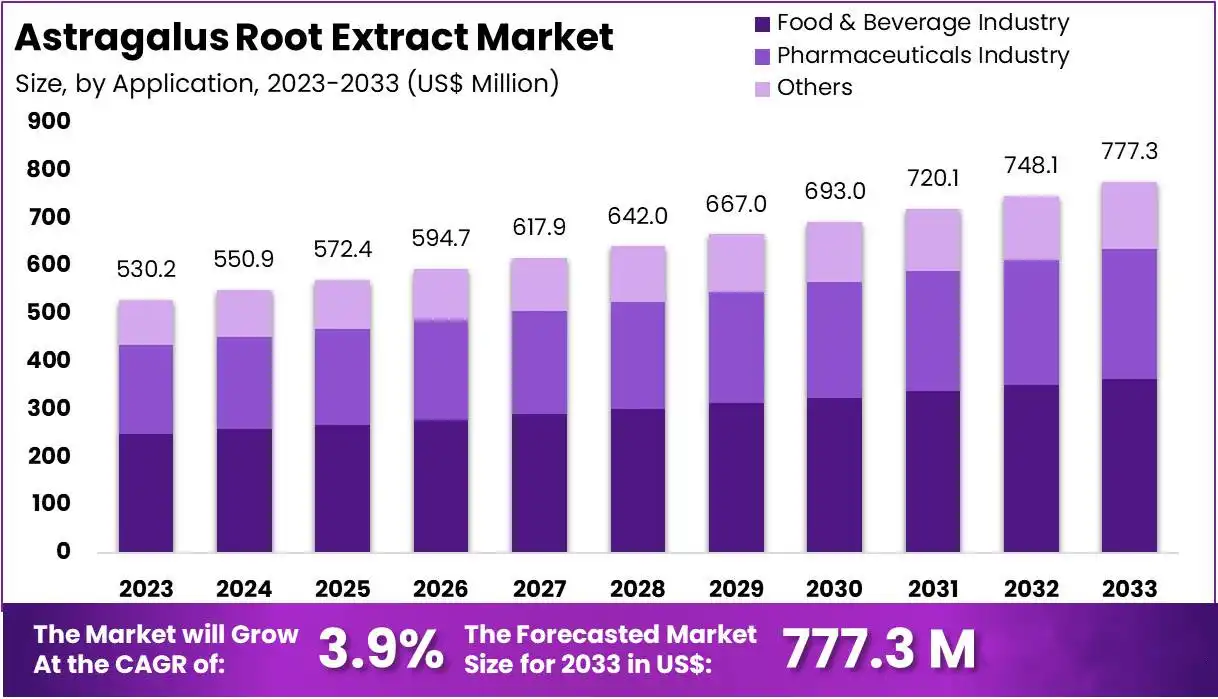
The global astragalus extract powder market is expanding significantly, propelled by rising demand from the nutraceutical and pharmaceutical industries. Manufacturers are increasingly incorporating this natural ingredient into dietary supplements, traditional herbal formulations, and functional foods. This surge is largely driven by growing consumer preference for natural and preventative health solutions, particularly those supporting immune function. Suppliers capable of adhering to strict quality standards and ensuring a consistent supply are optimally positioned to capture opportunities in this evolving marketplace.
Technological Advancements in Extraction Methods
Innovations in extraction technology are critically influencing the organic astragalus root extract powder segment. Advanced techniques enable suppliers to produce extracts with higher purity, improved bioavailability, and greater stability. Companies investing in research and development are better equipped to deliver innovative products that align with evolving industry requirements. Furthermore, these modern methods often support more sustainable operations and cost-efficient production, offering mutual benefits to both suppliers and manufacturers in the long term.
Shift Towards Sustainable and Ethically Sourced Ingredients
A noticeable transition toward sustainability and ethical sourcing is emerging within the botanical extract supply chain, including astragalus. Manufacturers now prioritize suppliers who demonstrate eco-friendly cultivation, responsible harvesting, fair trade agreements, and reduced environmental impact. This shift mirrors increasing consumer awareness and demand for products that are both ecologically sound and socially responsible. Suppliers able to provide verified certifications and transparent ethical practices are likely to gain a competitive edge in the market.
Conclusion
Choosing a trusted astragalus extract powder supplier requires careful consideration of quality standards, third-party certifications, and global market trends. By prioritizing suppliers who meet rigorous quality criteria, offer transparent documentation, and align with industry best practices, manufacturers can ensure a reliable source of high-quality astragalus extracts. As the market continues to evolve, partnering with forward-thinking suppliers who invest in sustainable practices and innovative technologies will be crucial for long-term success in the astragalus-based product industry.
Contact Us
For more information about our premium astragalus extract powder and to discuss your specific requirements, please contact our expert team at sales1@bovlin.com. As a leading astragalus root powder manufacturer and supplier, we are committed to providing top-quality organic astragalus root extract to meet your production needs.
References
World Health Organization (2007). WHO Guidelines on Good Manufacturing Practices (GMP) for Herbal Medicines. Geneva: WHO Press.
International Organization for Standardization (2018). ISO 22000: Food Safety Management Systems - Requirements for Any Organization in the Food Chain. Geneva: ISO.
Upton, R., Graff, A., Jolliffe, G., Länger, R., Williamson, E. (2011). American Herbal Pharmacopoeia: Astragalus Root (Astragalus membranaceus) Monograph. American Herbal Pharmacopoeia, Scotts Valley, CA.
Li, X., Qu, L., Dong, Y., Han, L., Liu, E., Fang, S., Zhang, Y. (2014). A review of recent research progress on the astragalus genus. Molecules, 19(11), 18850-18880.
Smith, T., Gillespie, M., Eckl, V., Morton, C., & Henley, C. (2020). Sales of herbal dietary supplements in the United States increase by 9.4% in 2019. HerbalGram, 127, 54-69.
United States Pharmacopeia (2020). USP-NF Monographs: Dietary Supplements - Botanical Extracts. Rockville, MD: United States Pharmacopeial Convention.











