Regulatory Guidelines and Safety Assessments for Hydrolyzed Wheat Protein
Global Regulatory Framework
The cosmetics industry operates under stringent regulatory frameworks designed to ensure product safety. In the context of hydrolyzed wheat protein, various global authorities have established guidelines and safety standards. The European Union's Cosmetic Regulation, the United States Food and Drug Administration (FDA), and Japan's Ministry of Health, Labour and Welfare have all evaluated hydrolyzed wheat protein and provided regulatory guidance for its use in cosmetics.
These regulatory bodies typically assess factors such as the molecular weight distribution of the hydrolyzed protein, the hydrolysis process used, and potential contaminants. They also consider the intended use of the product and the expected exposure levels. Manufacturers must stay abreast of these regulations, which may vary by region, to ensure compliance and product safety.
Safety Assessments and Toxicological Studies
Numerous safety assessments and toxicological studies have been conducted on hydrolyzed wheat protein. These studies evaluate various aspects of safety, including potential for skin irritation, sensitization, and systemic toxicity. The Cosmetic Ingredient Review (CIR) Expert Panel, an independent body of scientific and medical experts, has reviewed the safety of hydrolyzed wheat protein and concluded that it is safe for use in cosmetics when formulated to be non-sensitizing.
Toxicological studies typically involve in vitro and in vivo testing to assess potential adverse effects. These studies have generally shown that hydrolyzed wheat protein has a low potential for toxicity when used as directed in cosmetic formulations. However, it's crucial to note that safety can depend on factors such as the specific hydrolysis process used and the molecular weight distribution of the resulting protein fragments.
Quality Control Measures
Ensuring the safety of hydrolyzed wheat protein in cosmetics begins with rigorous quality control measures. Manufacturers must implement strict protocols to monitor the quality and consistency of their hydrolyzed wheat protein powder. This includes regular testing for contaminants, verifying the molecular weight distribution, and ensuring batch-to-batch consistency.
Quality control also extends to the sourcing of raw materials. Wheat used for hydrolysis should be of high quality and free from pesticides and other contaminants. The hydrolysis process itself must be carefully controlled to achieve the desired protein fragmentation while avoiding the creation of potentially allergenic peptides.
Potential Irritants and Allergenic Risks in Cosmetic Use
Understanding Wheat Allergies and Sensitivities
While hydrolyzed wheat protein is generally considered safe, it's important to acknowledge the potential risks associated with wheat allergies and sensitivities. Wheat allergies are relatively rare, affecting approximately 0.4% of adults. However, for those with wheat allergies, exposure to wheat proteins, including hydrolyzed forms, can trigger allergic reactions.
It's crucial to distinguish between wheat allergy and celiac disease. Celiac disease is an autoimmune disorder triggered by gluten, a protein found in wheat. While hydrolyzed wheat protein is not the same as gluten, individuals with celiac disease or gluten sensitivity may still have concerns about using products containing wheat-derived ingredients.
Molecular Weight and Allergenic Potential
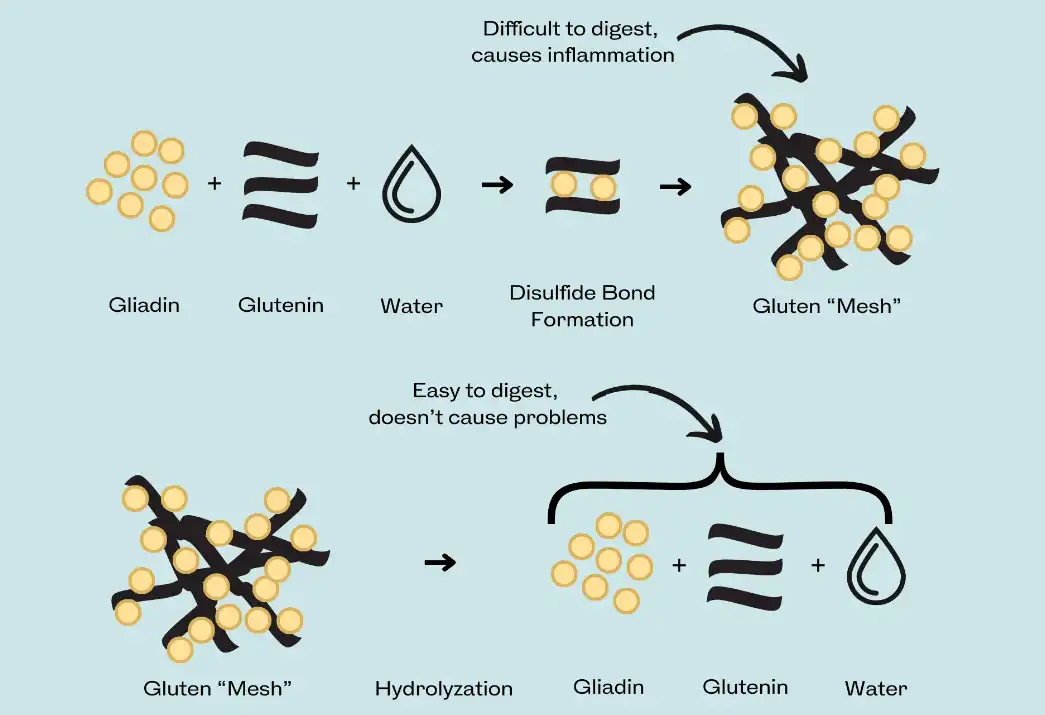
The allergenic potential of hydrolyzed wheat protein, also known as wheat peptide, is closely linked to its molecular weight. Proteins with higher molecular weights are more likely to trigger allergic responses. During the hydrolysis process, wheat proteins are broken down into smaller peptides. The degree of hydrolysis and the resulting molecular weight distribution play a crucial role in determining the potential for allergic reactions.
Regulatory bodies often set guidelines for the maximum molecular weight of hydrolyzed proteins used in cosmetics. For example, the Japanese government recommends that the weight-average molecular weight of hydrolyzed wheat protein in cosmetics should not exceed 3,500 Daltons. This guideline aims to minimize the risk of sensitization and allergic reactions.
Identifying and Mitigating Risks
To address potential allergenic risks, manufacturers must implement comprehensive risk assessment and mitigation strategies. This includes thorough testing of raw materials and finished products, as well as clear labeling practices to inform consumers about the presence of wheat-derived ingredients.
Patch testing and human repeat insult patch tests (HRIPT) are common methods used to assess the potential for skin irritation and sensitization. These tests help identify any adverse reactions before products reach the market. Additionally, manufacturers should consider alternative ingredients for products intended for individuals with known wheat allergies or sensitivities.
Best Practices for Formulating with Hydrolyzed Wheat Protein
Optimizing Molecular Weight Distribution
When formulating with hydrolyzed wheat protein, achieving the optimal molecular weight distribution is crucial. Lower molecular weight peptides are generally less likely to cause allergic reactions and can penetrate the skin more effectively, providing better moisturizing and conditioning benefits. However, extremely low molecular weight peptides may not provide the desired functional properties.
Manufacturers should work closely with suppliers to obtain hydrolyzed wheat protein with a well-characterized molecular weight distribution that balances safety and efficacy. Regular testing and quality control measures should be implemented to ensure consistency across batches.
Concentration and Formulation Considerations
The concentration of hydrolyzed wheat protein in a formulation can impact both its efficacy and safety profile. While higher concentrations may provide enhanced benefits, they also increase the risk of irritation or sensitization in some individuals. Manufacturers should carefully consider the intended use of the product and the target consumer when determining appropriate concentrations.
Formulation pH is another critical factor to consider. Hydrolyzed wheat protein typically performs best in slightly acidic to neutral pH ranges. Extreme pH values can affect the stability and performance of the ingredient, potentially leading to reduced efficacy or increased risk of irritation.
Compatibility with Other Ingredients
When incorporating hydrolyzed wheat protein into cosmetic formulations, it's essential to consider its compatibility with other ingredients. While hydrolyzed wheat protein is generally stable and compatible with a wide range of cosmetic ingredients, certain combinations may affect its performance or stability.
For example, high concentrations of electrolytes or certain preservatives may impact the solubility or stability of hydrolyzed wheat protein. Manufacturers should conduct thorough stability testing to ensure that the inclusion of hydrolyzed wheat protein does not compromise the overall stability or efficacy of the formulation.
Conclusion
Hydrolyzed wheat protein remains a valuable ingredient in the cosmetics industry, offering numerous benefits for hair and skin care products. While safety concerns exist, particularly regarding potential allergenic risks, proper formulation practices and adherence to regulatory guidelines can mitigate these risks effectively. By implementing rigorous quality control measures, optimizing molecular weight distributions, and carefully considering formulation parameters, manufacturers can harness the benefits of hydrolyzed wheat protein while ensuring product safety. As the cosmetics industry continues to evolve, ongoing research and regulatory oversight will further refine our understanding of this versatile ingredient, enabling the development of even safer and more effective products.

Contact Us
For more information about our high-quality hydrolyzed wheat protein powder and expert formulation support, please contact us at sales1@bovlin.com. Our team of specialists is ready to assist you in creating safe, effective, and innovative cosmetic products.











